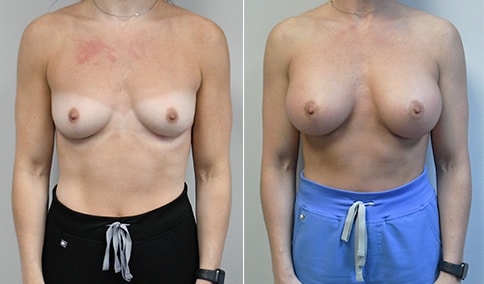
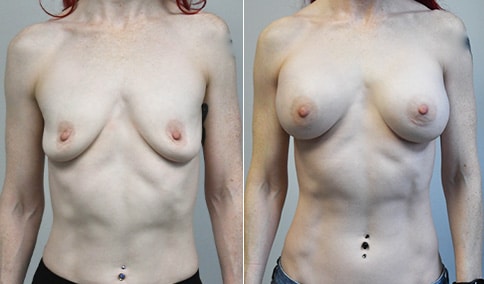
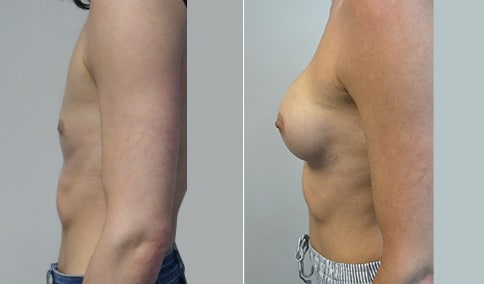
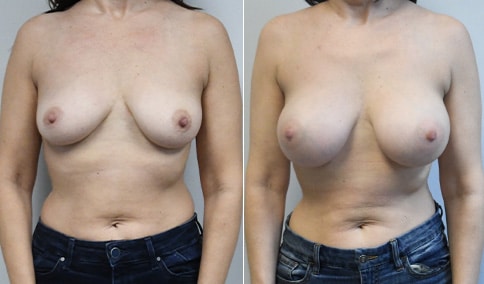
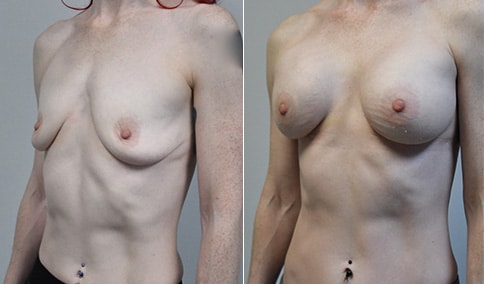
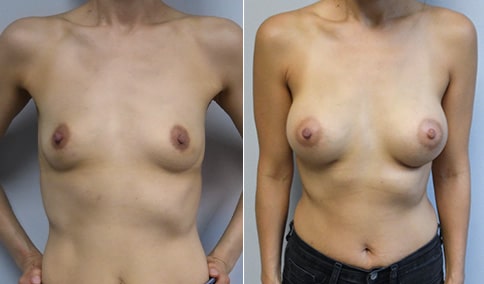
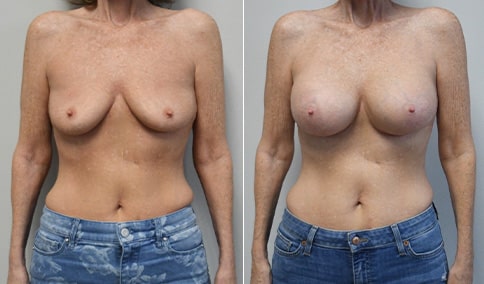
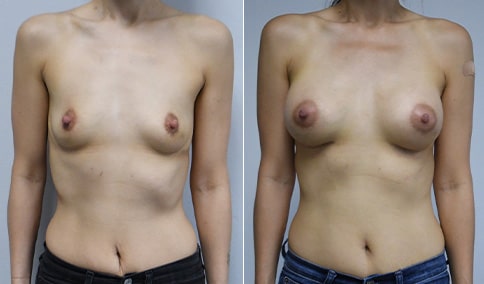
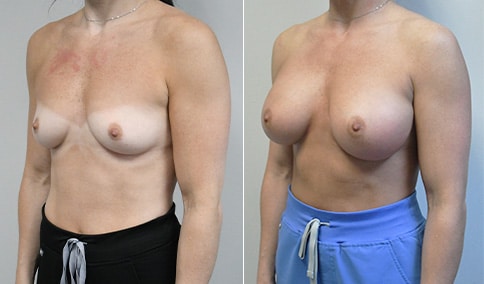
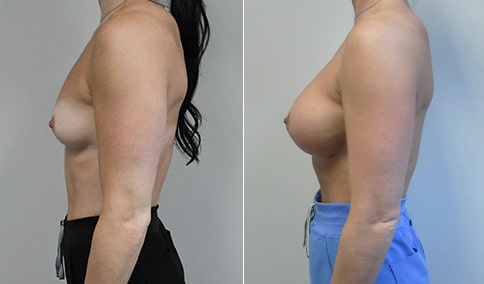
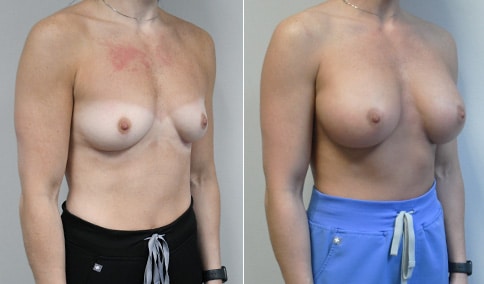
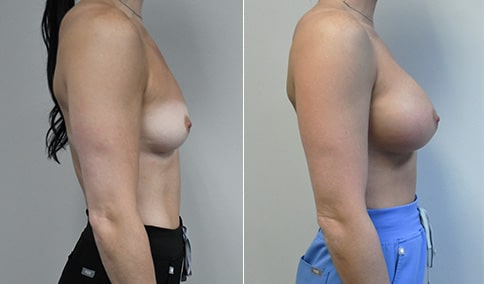
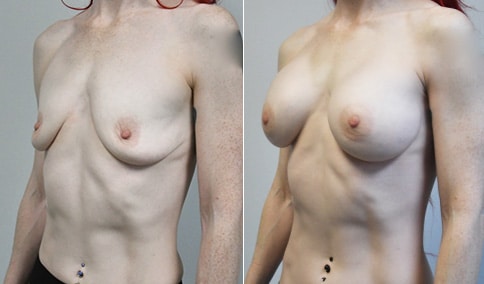
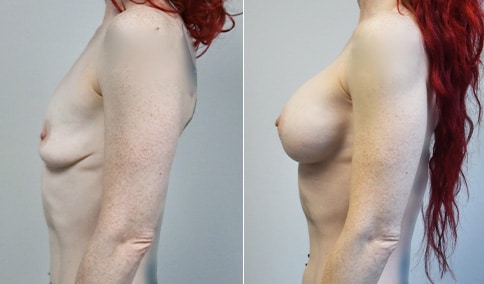
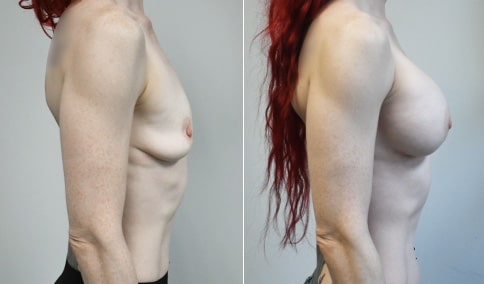
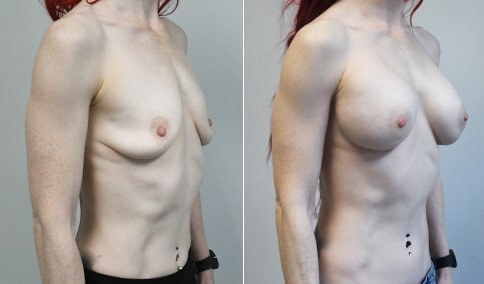
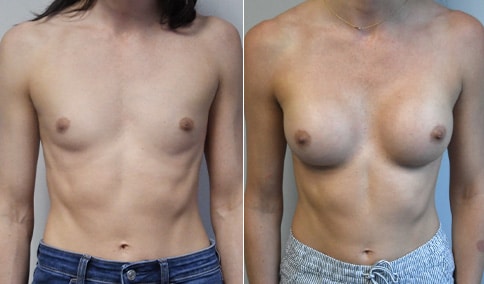
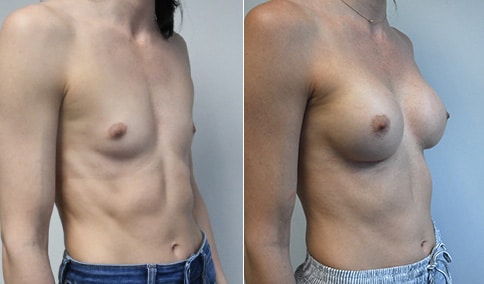
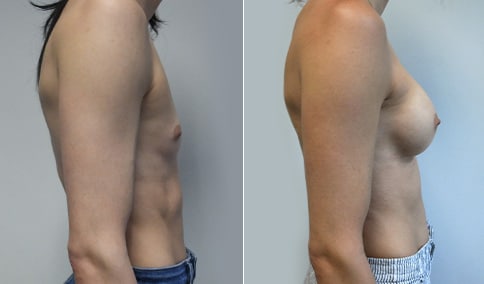
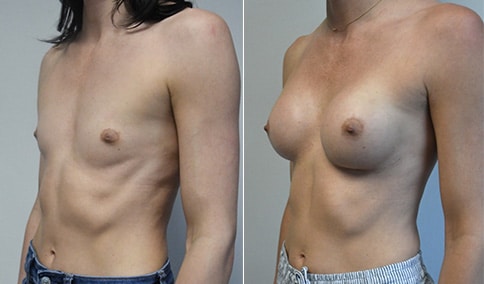
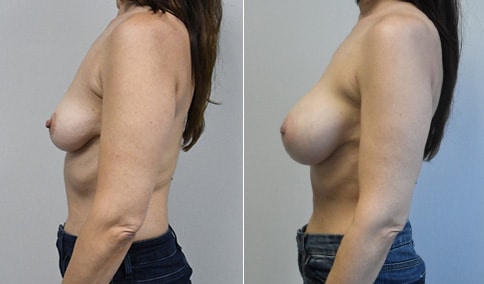
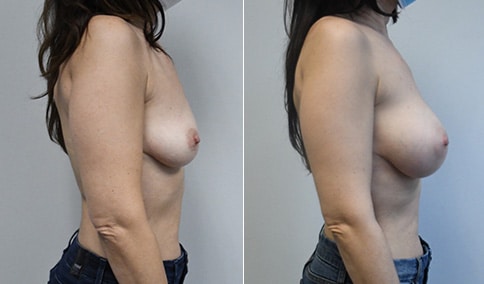
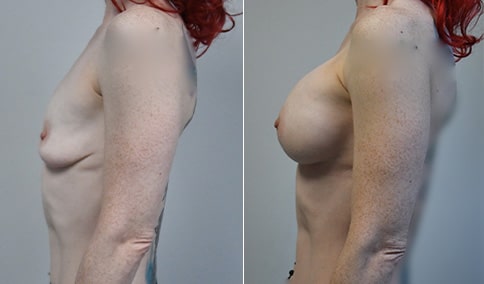
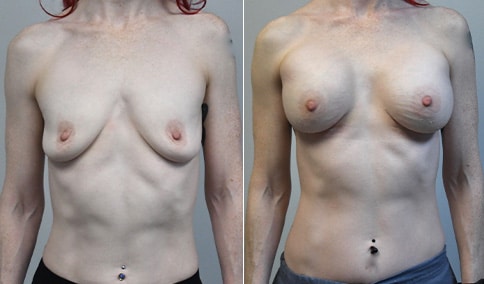
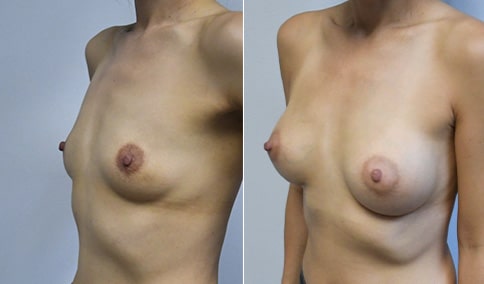
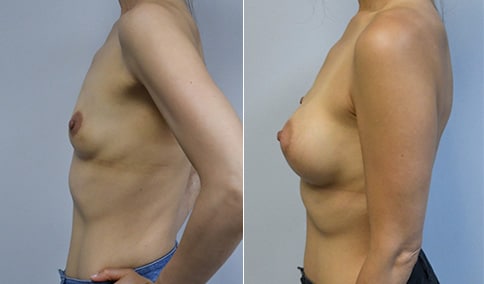
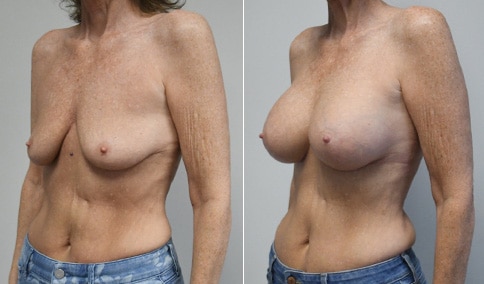
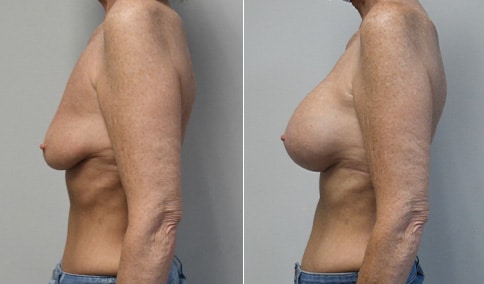
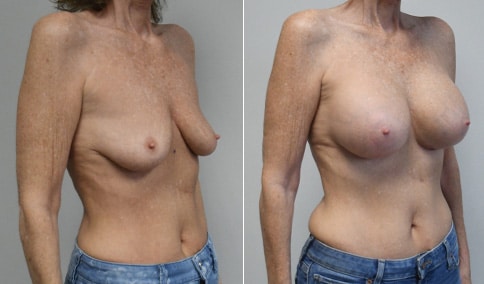
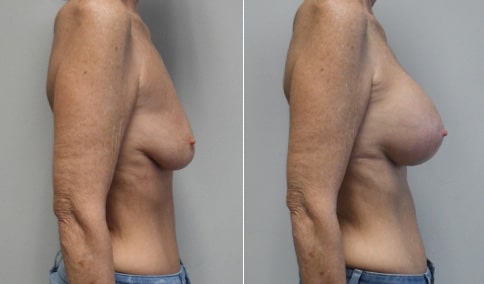
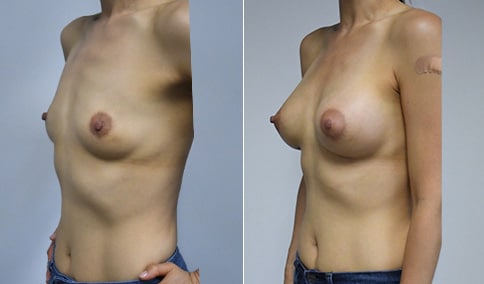
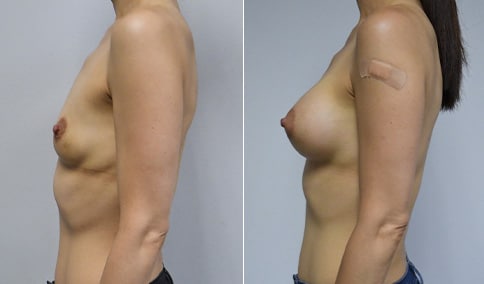
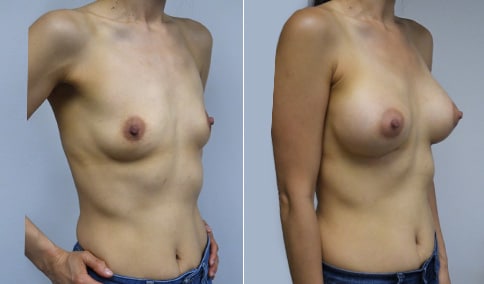
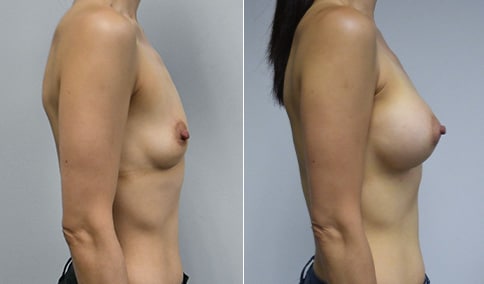
A mother of 4 in her mid-30s who is bothered by the deflated appearance of her breasts after pregnancies. She is shown before and again, 6 weeks after bilateral partial subpectoral breast augmentation with smooth round silicone implants placed through an inframammary incision.
Discussion: Our patient is 5’5” and 132 lbs During our sizing appointment she liked the 375 and 400 and felt that 425 was starting to look too full. After discussions, she elected the Sientra 415 smooth strength cohesive moderate plus profile round silicone gel implants.
She had some asymmetry before surgery with her left breast fold being 5 mm higher than her right. Her measured breast base width is 13.1 cm on her right and 13 cm on her left, and her nipple to breast fold on stretch is 10.5 cm on each side. This implant has a base width of 12.95 cm and fits easily in the footprint of her breast, so she will not have an “implant” look.
She loves her result and like many women says she “could have gone bigger” but we know that on the sizing appointment, a larger implant would start to look unnatural for her frame, as well as having a higher risk of stretching and displacement.





Cosmetic & Plastic Surgery Specialist
"I treat my patients like I would treat
- Jonathan D. Hall, MD, FACSmembers of my own family."
Schedule Consultation