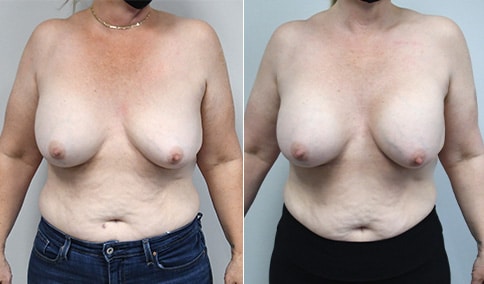
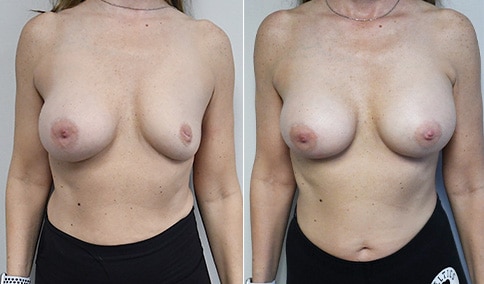
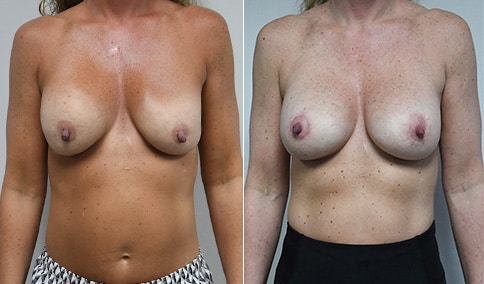
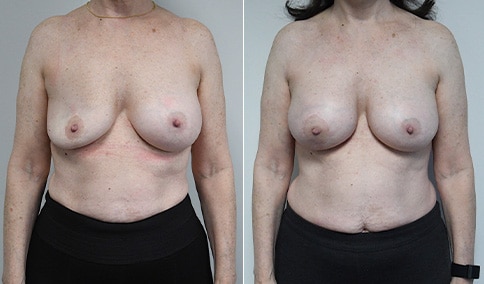
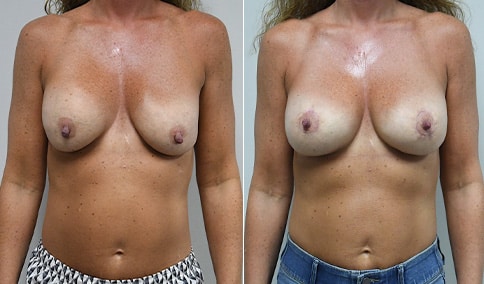
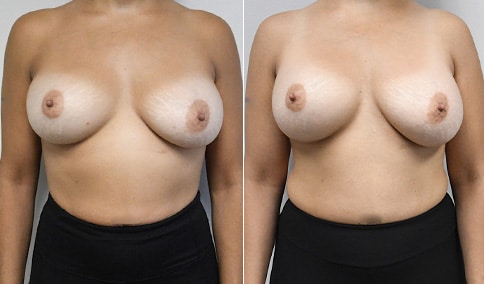
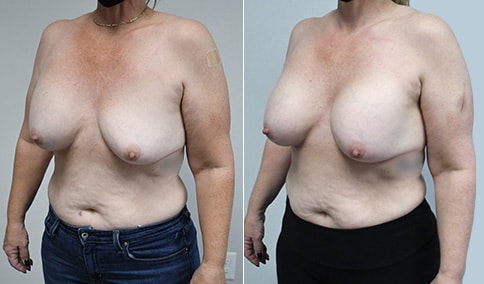
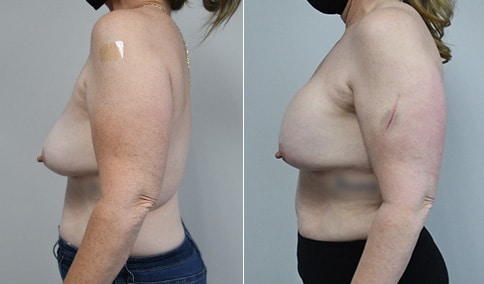
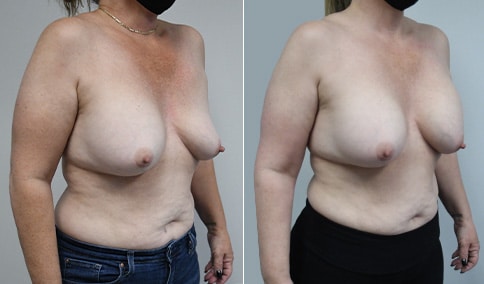
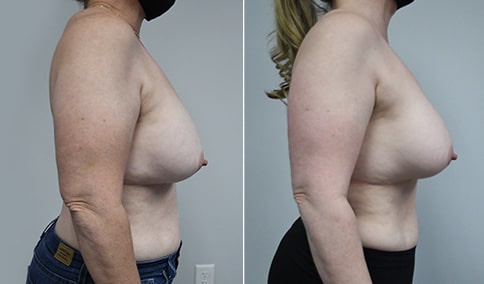
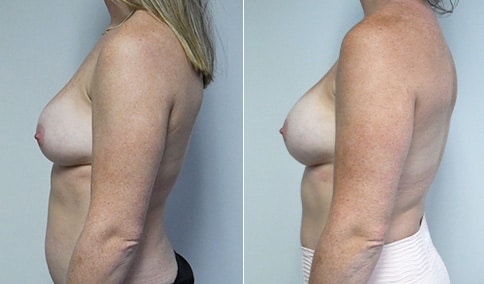
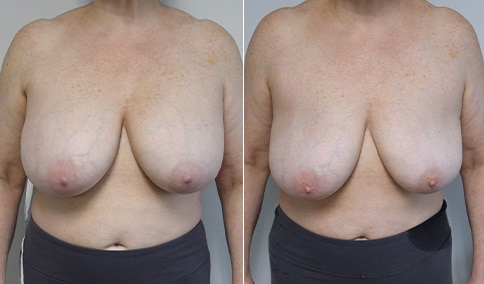
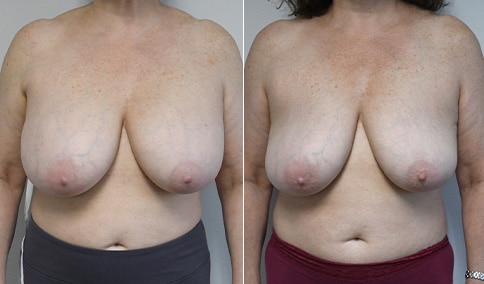
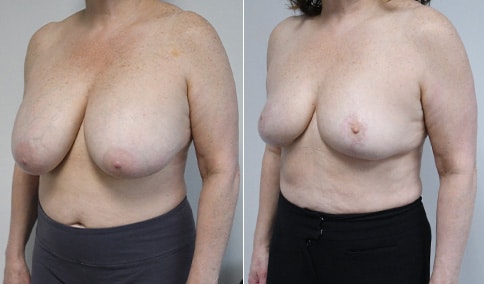
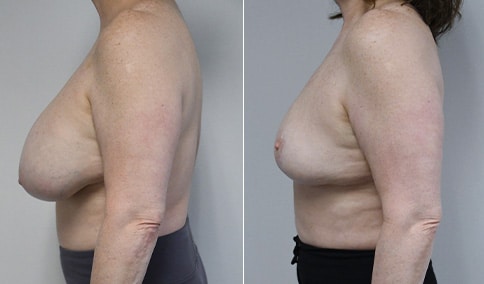
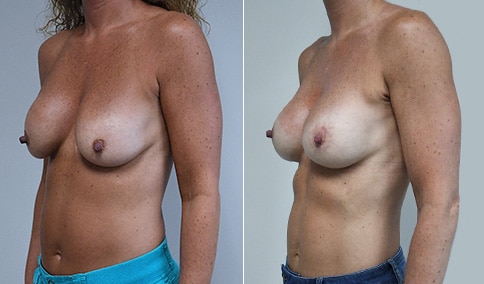
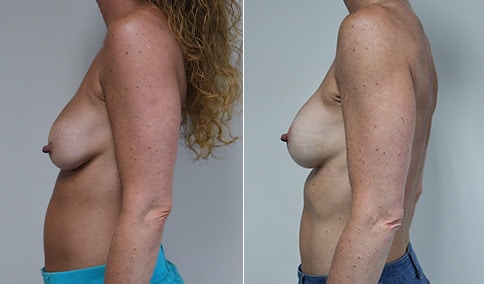
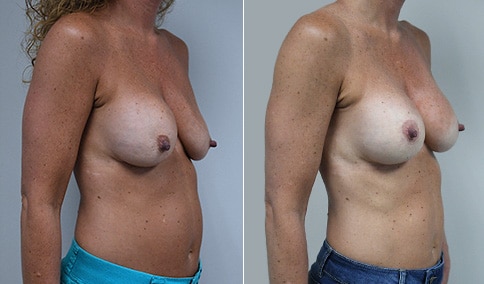
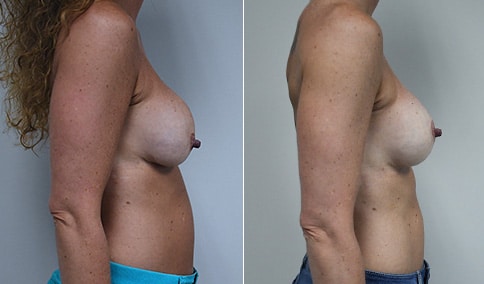
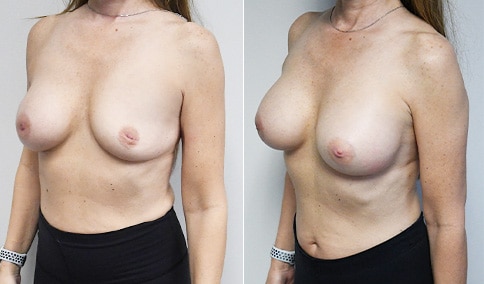
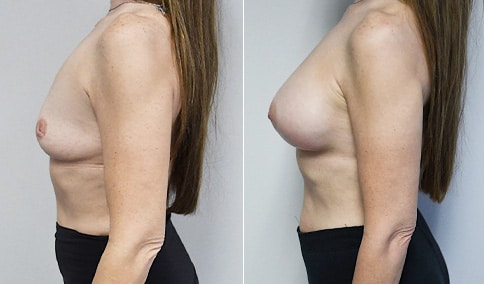
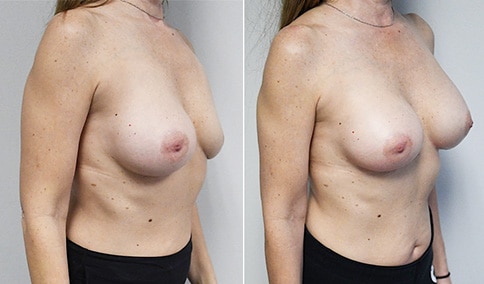
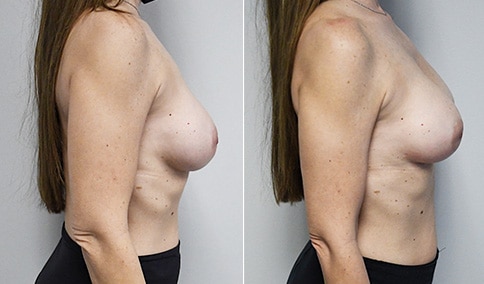
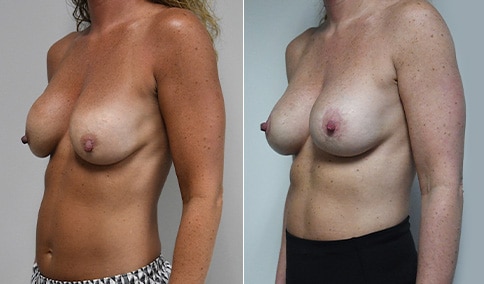
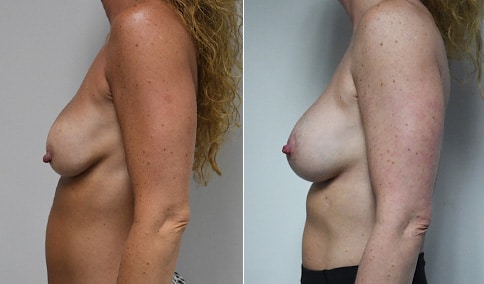
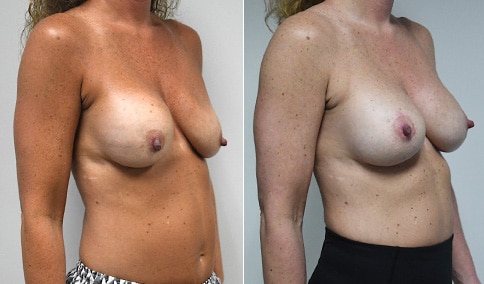
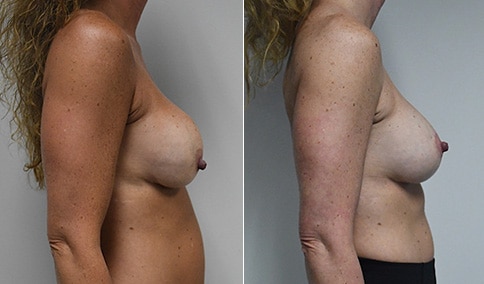
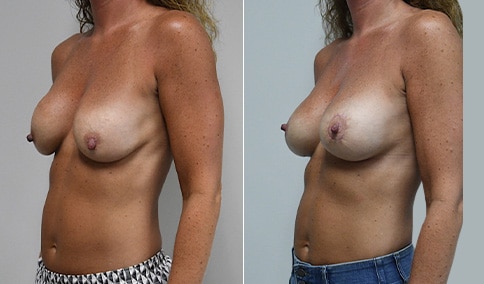
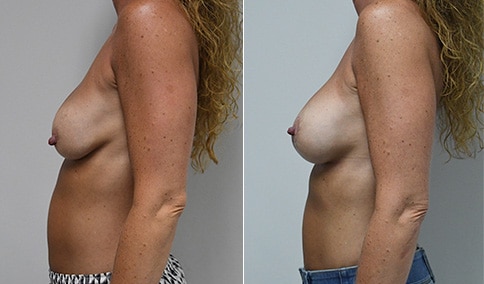
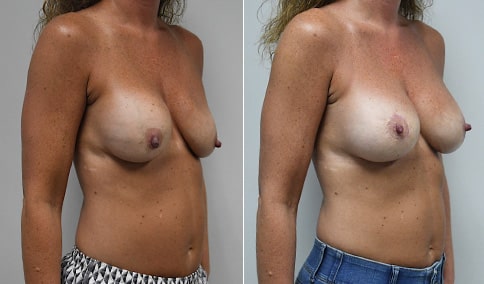
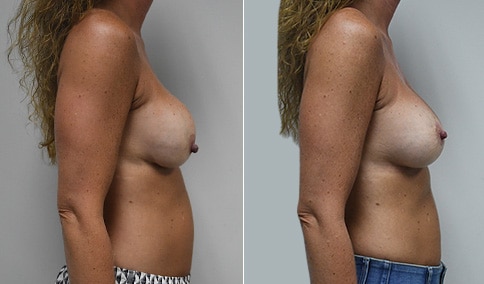
A woman in her mid-30s developed secondary fullness of her breasts after an initial breast reduction with us 14 years before. She is very petite at 5 feet tall and 119 lbs. She initially saw us in her early 20’s and was reduced from a DD to a “c” cup. She did well after her first surgery. Since that time her weight has fluctuated up and down and although she is back to her normal weight, her breasts are back to a 32 DD cup and she feels they are out of proportion to her frame. She has bulging of the upper breast/ axilla (“axillary lipodystrophy”). She is shown before and again, just 6 weeks after a secondary breast reduction with the removal of 160 grams of breast tissue from each breast along with liposuction of 150 cc from each breast.
Discussion:
An article in the Plastic Surgery Journal in June 2018 showed a retrospective review of patients undergoing a re-reduction by a single surgeon in Canada. The article was very similar to our experience here. The surgeon had Ninety patients who underwent breast re-reduction surgery using a random pattern blood supply to the nipple. ( which is what we did here) The average interval between the first and second surgery was 14 years ) the same here) with a range of 0-42 years. The majority of patients had undergone a primary reduction with an inferior pedicle technique. ( the same here) The nipple-areolar complex was repositioned in 60% of patients. (same here) The average volume of tissue resected was 250 grams with a range of 22-758 grams. ( our volume here was 160 grams of breast tissue and 150 ml of lipoaspirate from each breast ) Liposuction was used in all cases. ( as we did here)
The four key principles were:
1: the nipple-areolar complex can get elevated by deep laicization rather than recreating or developing a new pedicle
2. Breast tissue is removed where it is in excess, usually inferiorly and laterally ( which was done here)
3. The resection is complemented with liposuction to elevate the bottomed-out inframammary fold ( done here)
4. Skin should not be excised horizontally below the inframammary fold.
Our patient is thrilled with her 6-week result following the re-reduction of the breasts and will now start topical silicone to help with scar maturation. She is thrilled to be back to a C-cup





Cosmetic & Plastic Surgery Specialist
"I treat my patients like I would treat
- Jonathan D. Hall, MD, FACSmembers of my own family."
Schedule Consultation